seven. Do we really have to test systematically in parallel a preceding and authorised batch in an effort to Evaluate Using the new batch?
Violet Pink Bile Agar just isn't supposed to be used from the diagnosis of the disorder or other ailments in human beings.
Cooling and storage: Right after sterilization, enable the TSB containers to chill to area temperature before applying or storing them. Properly sealed containers of TSB might be saved at area temperature, from immediate daylight, for a specific length of time. However, it is suggested to use freshly organized or just lately sterilized TSB for optimal results.
This permits them to share in-depth expertise in The brand new e-book. Steven Brimble, Cherwell Laboratories’ Top quality Manager and Microbiologist feedback: “I hope this guidebook helps suggest end users on greatest procedures to aid them with fine-tuning their GPT procedures for their own personal website and testing wants.
Prior to a different batch of tradition media might be unveiled for almost any microbiological tests, particularly below pharmaceutical industry polices, it need to be appropriately tested for its power to ensure dependable and reputable effects. Growth promotion testing (GPT) is central to this.
You need to utilize the strains which might be cited Within this chapter, or equal strains from other culture collections. As an example, if Pseudomonas aeruginosa ATCC 9027 is indicated, you must use this strain or strains from other tradition collections proclaiming equivalence to ATCC 9027. Other strains for example ATCC 14149 will not be ideal.
Your environmental isolates may be skillfully characterised, preserved and created inside of a handy, All set-to-use format using a plan called Microbiologics Custom made Answers. Make contact with your Microbiologics profits agent if you want to more details about This system.
Ahead of the availability of large-excellent reference supplies, growth promotion testing was generally carried out by plating a serial diluted microorganism suspension on both of those a completely new along growth promotion testing with a Formerly unveiled media batch to check recoveries. This method proved complicated in acquiring exact outcomes
This chapter gives tests to show the usefulness of antimicrobial security. Extra antimicrobial preservatives has to be declared within the label. The tests and criteria for effectiveness apply to a product in the original, unopened container where it was dispersed via the producer.
For a offered product or service, When the antimicrobial activity with regard to the microorganism for which testing is prescribed cannot be neutralized, then it can be to generally be assumed website which the inhibited microorganism will not be present in the item.
Anresco Laboratories offers superb analytical companies that exceed my expectation! The two microbiology and chemistry departments go excess measures to assist fix the challenge and supply recommendations. Anonymous Responses
When the mouth from the vial is flamed, the pellets may very well be damaged and would most probably deliver reduced than envisioned counts on TSA agar.
TSB is made up of tryptone, soybean meal, dextrose, and various elements that supply a rich supply of nutrients essential for bacterial growth and metabolism.It supports the growth of a wide array of bacterial species, the two aerobic and anaerobic, making it a flexible medium.
Good media is appropriate when count will not be higher than 2 with the calculated price of the standardized value.
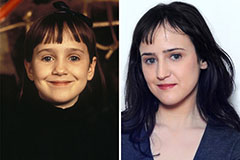 Mara Wilson Then & Now!
Mara Wilson Then & Now! Michael J. Fox Then & Now!
Michael J. Fox Then & Now! Val Kilmer Then & Now!
Val Kilmer Then & Now! Elisabeth Shue Then & Now!
Elisabeth Shue Then & Now! Heather Locklear Then & Now!
Heather Locklear Then & Now!